Introduction: Allogeneic stem cell transplantation (allo-SCT) for patients with relapsed/refractory Hodgkin lymphoma (r/R HL) after autologous transplantation is now an established option. Many patients receive Check-Point Inhibitors (CPI) as relapse treatment. Nevertheless, concerns have been previously reported on higher risk of acute graft versus host disease (aGVHD) after CPI exposure, without any control group to elucidate risk factors.
Methods: We conducted a multicentric retrospective case control study on behalf of the Francophone Society for Bone Marrow Transplantation and Cell Therapy to compare outcomes after allo-SCT for patients with r/R HL who were exposed or not exposed to CPI.
Results: Between 2015 and 2018, 149 patients underwent allo-SCT for r/R HL in 21 centers. CPI was used for 50 patients (n=48 Nivolumab, n=2 Pembrolizumab). We did not identify any differences regarding baseline characteristics. Reduced intensity conditioning (RIC) was commonly used (90.2%), with Peripheral Blood Stem Cells (75.7%). A majority of patients had haploidentical (44.6%), followed by siblings (28.4%), matched unrelated (24.3%) and mismatched unrelated donors (2.7%).
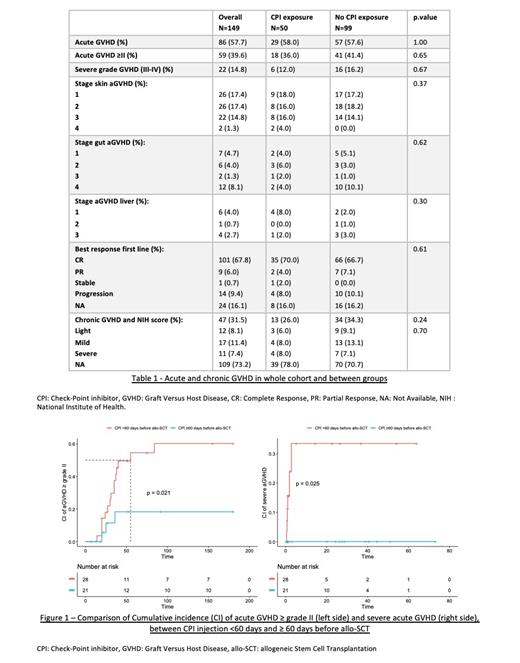
Cumulative incidence (CI) of aGVHD grade ≥II in the whole cohort was 39.6% and 14.8% developed a severe form (grade III-IV). There were no differences between CPI (n=50) and no CPI cohorts (n=99), regarding CI of aGVHD of any grade (respectively 58% and 57.6%, p=1.00), grade ≥II (respectively 36% and 41.4%, p=0.65), severe aGVHD (grade III-IV, respectively 12% and 16.2%, p=0.67) and chronic GVHD (respectively 26% and 34.3%, p=0.24), as described in Table 1. We did not observe excess of mortality from GVHD between both groups.
The time interval between the last CPI injection and allo-SCT was found to be the only significant factor associated with a higher incidence and severity of aGVHD, as depicted in Figure 1. The median time from the last CPI injection and allo-SCT was 50 days [33;103]. Specifically, within 30 days, the incidence of severe aGVHD was significantly increased at 41.7% compared to only 2.7% in the control group (p=0.002). No severe forms were observed after 60 days, but it persisted a higher risk of aGVHD grade ≥II even after 60 days of delay. Thus, a higher percentage of patients required systemic therapy for aGVHD within 120 days compared to after 120 days (45% vs 0% respectively, p=0.03). The delay of CPI treatment did not impact the CI and severity of chronic GVHD or CI of relapse after allo-SCT. Of note, post-transplant cyclophosphamide did not reduce CI of aGVHD. With a median follow-up of 34.7 months, the 2-year Overall Survival, Relapse Free Survival and GVHD free, Relapse Free Survival (GRFS) were respectively 69.9% (IC95 41.1-78.5), 67.1% (IC95 58.8-76.7) and 48.5% (IC95 40.3-58.4), without differences between CPI and no CPI cohorts. Two-year non relapse mortality was 17.4% in whole cohort, without hepatic sinusoidal obstruction syndrome nor steroid-requiring febrile syndrome in CPI cohort.
Conclusion: CPI use before allo-SCT within 60 days before allo-SCT can lead to significant severe aGVHD, with persisting higher risk of aGVHD grade ≥II even after 60 days of delay. This highlights the importance of exercising caution when administering CPI injections within 60 days before allo-SCT. There is need to develop GVHD prophylaxis strategies in this population at risk. The findings from this study may help inform clinical decision-making and management of HL patients undergoing allo-SCT.
Disclosures
Beauvais:Kite/Gilead: Honoraria, Other: Advisory Board; BMS: Honoraria, Other: Advisory Board. Forcade:Astellas: Speakers Bureau; Novartis: Consultancy, Other: Travel support, Speakers Bureau; Alexion: Other: Travel support, Speakers Bureau; Sanofi: Speakers Bureau; GSK: Speakers Bureau; Gilead Sciences: Other: Travel support, Speakers Bureau; MSD: Other: Travel support. Chevallier:Sanofi: Honoraria; Mallinckrodt Pharmaceuticals: Honoraria; Incyte: Honoraria, Research Funding; Takeda: Honoraria; Immedica Pharma: Honoraria; Servier: Honoraria. Loschi:Abbvie: Honoraria; Alexion: Honoraria; Astra Zeneca: Honoraria; BMS Celgene: Honoraria; Gilead: Honoraria; GSK: Honoraria; Jazz: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Sobi: Honoraria; Takeda: Honoraria. Huynh:Astellas: Other: Advisory board; Pfizer: Other: advisory board; Servier: Other: Advisory board; Medac: Other: Advisory board; Neovii: Other: Advisory board; Jazz: Other: travel fees, advisory board; Novartis: Other: travel fees, advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal